
SYYY020170A Equipment parameters
01. Basic parameters
Dimensions: L1262mm×W480mm×H790mm
Weight: 100Kg
Minimum site requirement: 1.3m×0.8m×1m
02. Powertrain Configuration
Power supply: AC220V ±10% 50Hz
Power: 1.8kW
Air source pressure: ≥0.6MPa
03. Process Capabilities
Applicable wire diameter range: 3-0# to 1# suture (USP standard)
Finished product length: ≥50mm
Production rhythm: ≤1.2s/thorn (material related)
04. Core Modules
Imprinting method: high-precision mold imprinting
Spiral type: fishbone spur-shaped barbs
Mold switching method: convenient modular design
05. Vision System
Industrial-grade microscope lens (magnification ≥50X)
Real-time monitoring: thorn-shaped forming quality
Product Overview
This equipment is a professional fishbone thread embossing and forming system developed for the high-end medical device field. It integrates precision machinery, intelligent control, and online visual inspection technologies, and is specifically designed for the continuous and high-precision processing of the fishbone-shaped barb structure of surgical sutures. Through a modular architecture, it realizes the closed-loop production of the entire process, including automatic feeding, precise tension control, multi-station embossing, fixed-length cutting, and finished product collection, meeting the stringent requirements of the ISO 13485 Medical Device Quality Management System for process stability. The system adopts a multi-threaded concurrent architecture and is equipped with a process logic editor with independent intellectual property rights, achieving the decoupling of motion control, process logic, and process scheduling.
Product characteristics
● High-precision imprinting process
High-precision mold forming technology is used to ensure the consistency of the fish bone shape.
Adapt to 3-0# to 1# wire diameter sutures.
Support dynamic tension compensation algorithm to adapt to the mechanical property differences of sutures of different materials.
● Modular intelligent system
The feeding, stamping and cutting mechanisms adopt standardized and modular designs, which meet the GMP modular assembly requirements of the medical device industry.
● Real-time quality monitoring
Use industrial-grade microscope lens, magnification ≥50X.
Real-time monitoring of thorn forming quality.
● Flexible production platform
Mold replacement time ≤ 5 minutes, compatible with multi-thorn process expansion, meeting small batch and multi-variety production needs.
The process database presets 3-0# to 1# specification fishbone wire process data, supporting user-defined process formulas.
Dongguan Fortune Medical Technology Co., Ltd. (Introduction to absorbable polymer materials)
Dongguan Fortune Medical Technology Co., Ltd. is based on medical monomers and medical degradable polymer polyester materials (biomaterials), with interventional non-implantable devices (absorbable devices) as the core, providing customers with integrated, End-to-end high-end consumables R&D and production services continue to lower the R&D threshold for absorbable medical devices and help customers improve R&D efficiency. Bring more breakthrough treatment plans to patients, and the service scope covers the research and development and production of medical monomers, medical degradable polymer polyester materials (biomaterials), medical absorbable monofilaments/multifilaments, and absorbable medical devices.
In terms of polymer degradable materials and equipment, our technical core is based on years of accumulation, with mature and mature technical reserves, and has become a pioneer and leader in China's absorbable materials market.
Our website is online: "www.fortunemedical-int.com", and some pages are still under construction. Welcome to our official website to purchase products.
-
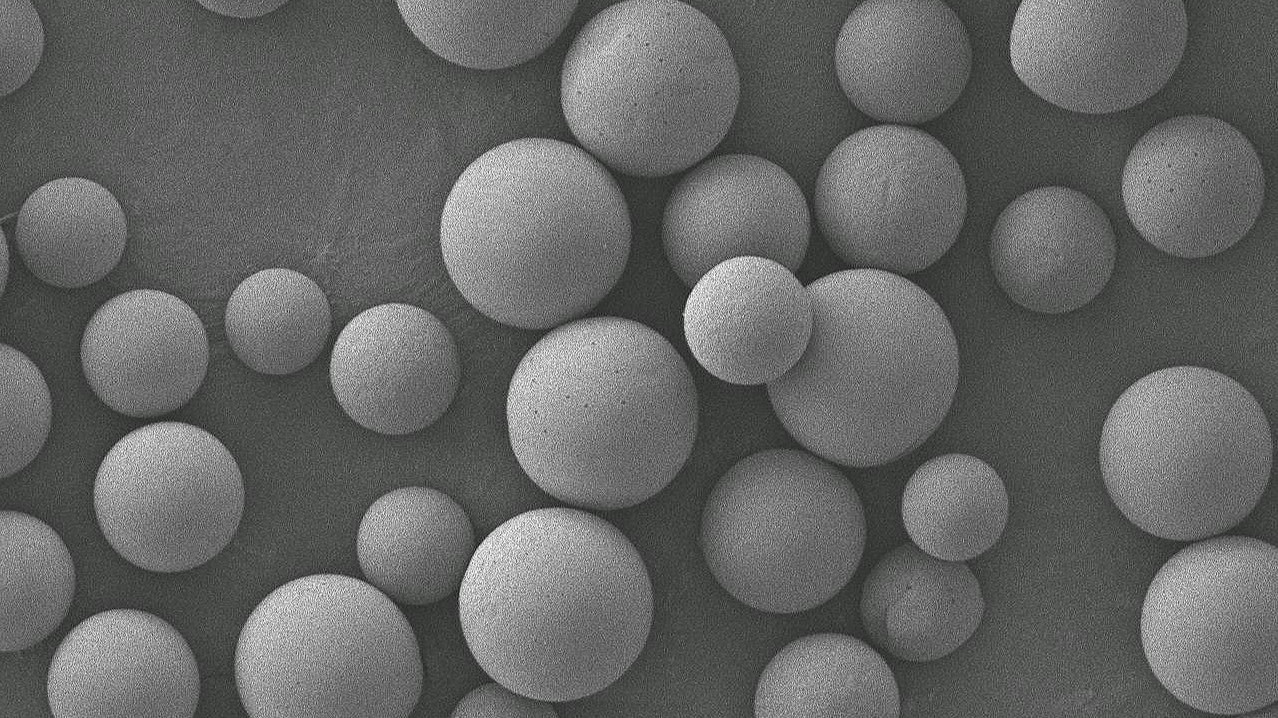
Quantitative Detection Method for Residual Monomer in Polylactic Acid
Polylactic acid has good biocompatibility and degradability, good thermoplasticity and high strength. It can be processed into various medical products. It has been widely used in medical device products such as fracture internal fixation and bone repair, degradable stents, absorbable sutures, tissue engineering stents, 3D printing implants, etc.2025-01-04
-
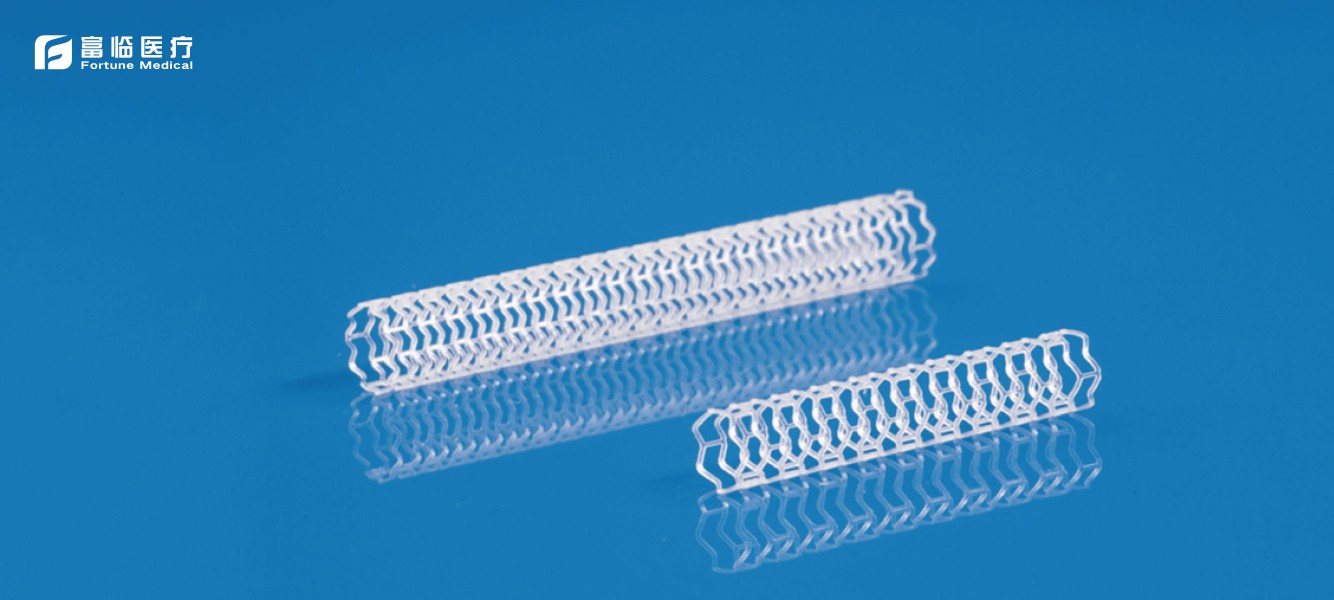
Application of biodegradable polymer materials in medical devices
The performance and degradation characteristics of several commonly used biodegradable polymer materials are reviewed, including polyglycolide,polylacticacid,(glycolide-lactide) copolymer,polycaprolactone,polydioxanone,polyhydroxyalkanoate, polytrimethylene carbonate, polyurethane and polyether urethane, etc., and their applications in medical devices, including implants, tissue engineering scaffolds, drug controlled release carriers, etc. are reviewed.2025-01-04
-

Method for determination of monomer residues in biodegradable polydioxanone materials
Establish a gas chromatography method for testing the residual monomer dioxanone in polydioxanone raw materials. A DB-624 capillary column (30.0 m ×535 μm ×3.00 μm) was used, and the temperature was programmed (146 ℃ for 5 min, 30 ℃/min to 200 ℃, and maintained for 2 min).2025-01-04





