The performance and degradation characteristics of several commonly used biodegradable polymer materials are reviewed, including polyglycolide,polylacticacid,(glycolide-lactide) copolymer,polycaprolactone,polydioxanone,polyhydroxyalkanoate, polytrimethylene carbonate, polyurethane and polyether urethane, etc., and their applications in medical devices, including implants, tissue engineering scaffolds, drug controlled release carriers, etc. are reviewed.
Biomaterials play an important role in disease treatment and medical care. According to the properties of the materials, biomaterials can be divided into two types: inert materials and degradable materials. The current development of biomaterials shows a trend of transition from inertness to degradability (hydrolysis and enzyme degradation), which indicates that many bio-inert devices that play a temporary therapeutic role (helping the body repair or regenerate damaged tissues) will be replaced by degradable materials. Compared with inert materials, degradable polymer materials are a more ideal medical device material. Inert devices generally have the problems of poor long-term compatibility and the need for secondary surgery, while degradable polymer materials do not have these defects. In the late 1960s, synthetic degradable polymer materials began to be used in clinical practice. In the past 20 years, some new medical technologies have emerged in biomedicine, including tissue engineering, drug controlled release, regenerative medicine, gene therapy and bio-nanotechnology. These new medical technologies all require degradable polymer materials as support, and they have also correspondingly promoted the development of degradable polymer materials.
Degradable polymer materials need to have good compatibility throughout the degradation process, mainly including the following points:
- No persistent inflammation or toxic reaction after implantation in the human body;
- Appropriate degradation cycle;
- During the degradation process, they have mechanical properties corresponding to the therapeutic or tissue regeneration function;
- The degradation products are non-toxic and can be excreted from the body through metabolism or osmosis;
- Processability. There are many factors that affect the biocompatibility of degradable polymer materials. Some properties of the material itself, such as the shape and structure of the implant, hydrophilicity and lipophilicity, water absorption, surface energy, molecular weight and degradation mechanism, need to be considered.
There are many kinds of medical polymer materials, each with its own characteristics, which are used to meet specific clinical needs. At present, there is no ideal universal medical polymer material. At present, the needs of different applications are mainly met by designing and synthesizing medical polymer materials with specific functions, which mainly include the following three methods:
- Designing and synthesizing polymer materials with unique functional groups;
- Biosynthesis of polymer materials with biomimetic structures;
- Using combinatorial computational methods to design new degradable polymer materials.
This paper reviews the performance and degradation characteristics of currently commonly used degradable polymer materials, as well as their applications in medical devices, including short-term implants, drug delivery carriers, and tissue engineering scaffolds.
1. Polyglycolide (PGA)
PGA is the earliest synthetic degradable polymer material used in clinical medicine. It has a high degree of crystallinity (45% to 55%). The high crystallinity gives it a large tensile elastic modulus. PGA is insoluble in organic solvents, with a glass transition temperature (Tg) between 35 and 40 ℃ and a melting point (Tm) above 200 ℃. It can be processed by extrusion, injection molding, and molding. Due to its good fiber-forming properties, PGA was first developed into an absorbable suture. In 1969, the first synthetic degradable suture DEXON® approved by the US FDA was made of PGA. Because PGA has suitable degradability, excellent initial mechanical properties and biological activity, PGA non-woven fabrics have been widely studied as tissue regeneration scaffold materials. Currently, a scaffold material containing PGA non-woven fabrics is being used in clinical trials. In addition, PGA dura mater substitutes are also being studied because they have the ability to help tissue regeneration and close the skin without sutures. The high crystallinity of PGA gives it excellent mechanical properties. Among the degradable polymer materials used clinically, self-reinforced PGA is the hardest, with a modulus close to 12.5GPa. Because of its good initial mechanical properties, PGA has also been developed as an internal fixation system (Biofix®). PGA degrades by random cleavage (hydrolysis) of ester bonds in the chain segments. Under hydrolysis, PGA experiences a decrease in mechanical properties within 1 to 2 months and a mass loss within 6 to 12 months. In the body, PGA degrades into glycine, which can be directly excreted from the body through urine or metabolized into CO2 and H2O. The high degradation rate, acidic and poorly soluble degradation products limit the application of PGA in biomedicine, but these shortcomings can be overcome by copolymerization with other monomers.
2. Polylactic acid (PLA)
Lactide (LA) is a chiral molecule with two stereoisomers: left-handed LA (L-LA) and right-handed LA (D-LA), and their homopolymers are semi-crystalline. Racemic LA (DL-LA) is a mixture of L-LA and D-LA, and its polymer is random. The crystallinity (0% to 37%) of poly-L-LA (PLLA) is determined by molecular weight and processing parameters, with a Tg of 60 to 65℃ and a Tm of about 175℃. Because its hydrophilicity is worse than that of PGA, its degradation rate is lower than that of PGA. PLLA has high tensile strength, low elongation at break, and high tensile modulus (close to 4.8GPa), and is an ideal medical load-bearing material, such as bone fixation devices. The PLLA bone fixation devices currently on the market include BioScrew®, Bio-Anchor®, MeniscalStinger®, etc. In addition, PLLA can also be made into high-strength surgical sutures. In 1971, PLLA surgical sutures were approved by the US FDA for marketing, and they have better performance than DEXON®. PLLA can also be used in other medical fields, such as ligament repair and reconstruction, drug-eluting stents, targeted drug delivery, etc. In 2007, the US FDA approved an injectable PLLA product (Sculptra®) for the treatment of facial fat loss or atrophy caused by human immunodeficiency virus (HIV). The degradation rate of PLLA is slow. It takes 2 to 5.6 years for high molecular weight PLLA to be completely degraded in the body. Factors such as crystallinity and porosity can affect its degradation rate. Under hydrolysis, PLLA shows a decrease in mechanical properties within 6 months, but it takes a long time before mass loss occurs. Therefore, in order to obtain better degradation performance, researchers copolymerize L-LA with GA or DL-LA. Resomer®LR708 is a random copolymer obtained by copolymerizing L-LA and DL-LA (mass ratio 70:30). PDLLA forms a random copolymer due to the random distribution of L-LA and D-LA, with a Tg between 55 and 60 ℃ and a significant decrease in strength, which is caused by the random arrangement of the molecular chains. Under hydrolysis, PDLLA shows a decrease in mechanical properties within 1 to 2 months and a mass loss within 12 to 16 months. Compared with PLLA, PDLLA has the characteristics of low strength and high degradation rate, and is an ideal material for drug delivery carriers and tissue regeneration scaffolds (low strength). PLA is degraded by random cleavage of ester bonds in the chain segments (hydrolysis), and the primary degradation product is lactic acid, which is a byproduct of normal human metabolism. Lactic acid can be further degraded into CO2 and H2O through the citric acid cycle.
3. Copolymer (PLGA)
Studies have found that when the mass ratio of LA to GA is between 25/75 and 75/25, PLGA is a random copolymer. Studies by R.A.Miller et al. have shown that PLGA with a mass ratio of LA to GA of 50/50 has the fastest degradation rate.
PLGA with different monomer mass ratios has been widely used in clinical practice. PLGA with the trade name Purasorb®PLG is a semi-crystalline copolymer, in which the mass ratio of LA to GA is 80/20; the mass ratio of L–LA to GA in the multi-strand suture Vicryl® is 10/90, and its upgraded version VicrylRapid® has also been launched on the market, and the upgraded version after irradiation degrades faster;
PANACRYL® is another commercial PLGA suture. In addition, PLGA is also used in other medical fields, such as mesh (VicrylMesh®), skin graft materials and dura mater substitutes. Tissue engineering skin grafts use VicrylMesh® as a scaffold material. The ester bonds in PLGA break due to hydrolysis, and its degradation rate is affected by many factors, such as the mass ratio of LA to GA, molecular weight, shape and structure of the material, etc. PLGA has the characteristics of easy processing and controllable degradation rate. It is approved by the US FDA for use in the human body and has been widely studied in the fields of controllable drug/protein delivery systems and tissue engineering scaffolds. PLGA has the effect of promoting cell adsorption and proliferation, which makes it have potential tissue engineering applications. Many studies have prepared micron-nanoscale PLGA three-dimensional scaffolds. Figure 1 lists three PLGA structures obtained by different methods.
Another important application of PLGA is drug carrier and targeted release. PLGA can exist in various forms such as microspheres, microcapsules, nanospheres and nanofibers. The release parameters of drugs can be controlled by adjusting the properties of PLGA. Because PLGA is an overall erosion degradation, that is, the surface and the interior are degraded at the same time, it is difficult to achieve the effect of zero-order release.
4. Polycaprolactone (PCL)
PCL is a semi-crystalline linear polyester obtained directly from the relatively cheap monomer ε-caprolactone (ε-CL) through ring-opening polymerization. PCL has good processability, is easily soluble in many organic solvents, and has low Tm (55-60℃) and Tg (-60℃). PCL has a very low tensile strength (23MPa) and a high elongation at break (700%). In addition, it can be copolymerized with a variety of polymers. The degradation cycle of PCL is 2-3 years, and it is often used as a long-term drug controlled release carrier, among which micron-nanoscale PCL drug transport carriers are in the research stage. PCL is also used as a tissue engineering scaffold material. H.Tseng et al. used three different methods to increase the hydrophilicity of PCL, and then blended it with polyethylene glycol (PEG) to form an anisotropic hydrogel fiber scaffold. The scaffold has good biocompatibility and controllable structure, and is a potential heart valve tissue engineering scaffold material. Zhao Jing et al. prepared micellar nanoparticles of PCL-PEG copolymers, which can be used as transport carriers for picropodophyllotoxin (anticancer drug). In vitro (37℃) and phosphate buffer (PBS, PH=7.4), 70% of the drug can be released in 96 hours, which is consistent with the Higuchi equation. Therefore, PCL-PEG copolymer nanoparticles containing PPP are expected to become injectable preparations. Because the degradation rate of PCL is very slow, in order to obtain a faster degradation rate, researchers have developed several types of copolymers containing PCL. Copolymerization of ε-CL with DL-LA can obtain a faster degradation rate. Similarly, ε-CL can also be copolymerized with GA to make surgical sutures, which have a lower hardness than PGA. The monofilament suture MONACRYL® is such a product. In addition, multi-block copolymers composed of ε-CL, LA, GA and PEG can be used in drug controlled release systems. They are mainly used as carriers of small and medium-sized bioactive molecules (SynBiosys®). B.J.Hong et al. discovered a method for preparing PCL-based small interfering RNA (siRNA) carriers. The preparation process is simple and convenient, and it has a significant inhibitory effect on tumor cell proliferation.
5. Polydioxanone (PDS)
Although PLA and PGA can be made into universal degradable multifilament sutures, multifilament sutures have a high risk of infection during use, and multifilament sutures also have large friction when penetrating tissues. Therefore, many researchers are looking for polymer materials suitable for making monofilament sutures. PDS is a degradable polymer material suitable for making monofilament sutures. In the 1980s, the first PDS monofilament suture PDS® was launched on the market. In addition, PDS fixation screws (Orthosorb Absorbable Pins®) are also used in orthopedics, which are mainly used for the fixation and repair of small bones and cartilage. PDS is a colorless semi-crystalline polymer that can be obtained by ring-opening polymerization of p-dioxanone with a Tg of –10 to 0℃. As a member of polyester, its degradation is also achieved by random cleavage of ester bonds in the chain. High crystallinity and hydrophilicity give PDS a moderate degradation rate. In the body, PDS degrades into glyoxylic acid, which can be excreted from the body through urine, and can also be further degraded into glycine, which is consistent with the degradation product of GA. Compared with PGA, the tensile elastic modulus of PDS (close to 1.5 GPa) is very low. Under hydrolysis, the mechanical properties of PDS decrease within 1 to 2 months and the quality loss occurs within 6 to 12 months.
6. Polyhydroxyalkanoates (PHA)
PHA-based biodegradable polymer materials include poly 3-hydroxybutyrate (PHB), poly 4-hydroxybutyrate (P4HB), copolymers of PHB and poly 3-hydroxyvalerate (PHBV), etc. Among them, PHB is the most widely used. In 1920, researchers first discovered that Bacillus megaterium could produce PHB. Since then, studies have found that several other strains can also produce PHB. PHB is a semi-crystalline isotactic polymer with a melting point between 160 and 180℃. The degradation of PHB belongs to surface erosion degradation, which is different from the common overall erosion degradation. In addition to the bacterial preparation method, researchers have also developed a chemical synthesis process. B. Panchal et al. prepared PHB from the monomer β-butyrolactone through a ring-opening polymerization reaction, which is equivalent to PHB produced by bacteria. The copolymer of 3-hydroxybutyrate (HB) and 3-hydroxyvaleric acid (HA), P(HB–HV), has a semi-crystalline structure similar to PHB, and its Tg is -5~20℃. The Tm of P(HB–HV) decreases to different extents with the HV content. PHB and P(HB–HV) are easily soluble in organic solvents and can be easily processed into products of various shapes and structures. Because P(HB–HV) is less brittle, it is more suitable for use in biomaterials. In addition, P(HB–HV) has piezoelectric properties, which makes it applicable to orthopedics, because electrical stimulation can promote bone healing. PHB can achieve zero-order release when used as a drug transport carrier, but its degradation cycle is relatively long. In order to improve its degradation performance, researchers often copolymerize it with hydrophilic substances, generally PEG. A.V.Murueva et al. prepared a series of PHA microspheres as drug delivery carriers. The drug loading of the microspheres affects the size and zeta potential of the microspheres. After drug loading, the zeta potential decreases and the average diameter of the microspheres increases. The prepared PHA series of microspheres have excellent biocompatibility. PHA-based degradable polymer materials have potential uses in anti-infection. Studies have shown that PHA drug delivery systems can provide and maintain appropriate antibiotic solubility at the site of infection. PHA has been widely used in medical devices, cardiovascular tissue engineering, nerve conduit tissue engineering, bone tissue engineering, cartilage tissue engineering, drug delivery carriers and medical care.
7. Polytrimethylene carbonate (PTMC)
PTMC is an aliphatic polyester elastomer with low mechanical strength. It is usually used as a soft tissue regeneration scaffold or drug delivery carrier. The degradation rate of PTMC in vivo and in vitro varies greatly. It does not degrade within 2a in phosphate buffer (pH=7.4), but it is implanted in the subcutaneous tissue of the mouse back and degrades quickly. The main manifestation is that the shape of PTMC changes, but its molecular weight does not change, indicating that PTMC undergoes surface erosion degradation in vivo. PTMC with different molecular weights has different degradation rates. The degradation rate of high molecular weight PTMC is much faster than that of low molecular weight PTMC. This may be because low molecular weight PTMC is more hydrophilic. The hydrophilic surface reduces the activity of lipase and slows down the degradation rate. Zeng Ni et al. prepared PTMC barrier film for guiding bone regeneration in oral and maxillofacial surgery. Compared with collagen film, PTMC film can induce the formation of more bone tissue. In order to improve the penetration of drugs through the blood-brain barrier and improve the concentration of drugs in glioma cells, Jiang Xinyi et al. prepared 2-deoxy-D-glucose modified PEG-PTMC copolymer nanoparticles. The particles have an ideal size (71nm) with uniform distribution, high encapsulation efficiency and appropriate paclitaxel loading. In vitro and in vivo tests show that the particles have excellent blood-brain barrier penetration ability and targeting effect on intracranial tumor cells. The mechanical properties of PTMC are poor. Researchers often copolymerize it with other linear lactones to improve its mechanical properties. However, PTMC and its copolymers have good degradability and biocompatibility. R.A.Wach et al. prepared a porous catheter stent mixed with PLLA-trimethylene carbonate (TMC) copolymer and methylcellulose (MC), in which MC was used as a carrier of bioactive substances (such as growth factors). The results of physical and chemical properties and toxicity tests showed that the catheter is very suitable for nerve conduit regeneration, which can be used for the regeneration of the peripheral nervous system after injury. The copolymer of GA and TMC has been developed into flexible sutures (Maxon®) and orthopedic fixation devices (Acufex®). In addition, copolymerization of GA, TMC and dioxane can produce low-rigidity copolymers with a degradation cycle of 3 to 4 months, which can be used to make sutures (BioSyn®).
8. Polyurethane (PUR) and polyether urethane (PEU)
Non-degradable PUR and PEU have good biocompatibility and mechanical properties and can be used to make long-term medical implants, such as pacemakers and artificial blood vessels. Because non-degradable PUR has good biological properties and diverse synthetic pathways, researchers began to try to develop degradable PUR. PUR is generally prepared by the polycondensation reaction of diisocyanates and diols/diamines, but common diisocyanates such as 4,4'-diphenylmethane diisocyanate (MDI) and toluene-2,4-diisocyanate (TDI) are too toxic, so researchers have developed other aliphatic diisocyanates [such as 1,4-butane diisocyanate (BDI), hexamethylene diisocyanate (HDI), succinyl chloride (LDI), isophorone diisocyanate (IPDI) and lysine triisocyanate, etc.] to synthesize degradable PUR. LDI reacts with DL-LA, CL and other monomers to prepare degradable PEU, and its properties can be adjusted in a wide range. In these PEU, aliphatic polyesters constitute the soft segment and polypeptides constitute the hard segment. J.Podporska-Carroll et al. prepared a three-dimensional porous scaffold of poly(ester-urethane) urea (PEUU) using phase reversal technology, and inoculated human osteosarcoma MG-63 cells into the scaffold and cultured for 4 weeks. The results showed that the scaffold has the function of supporting cell adsorption, growth and proliferation, and is a potential cancellous bone substitute. J.R.Martin et al. prepared a selectively degradable polythioketal urethane (PTK-UR) tissue engineering scaffold, which can be selectively degraded by reactive oxygen species (ROS) produced by cells, thereby achieving coordination between tissue growth and material degradation. ROS is a key mediator for regulating cell function, especially in inflammation and tissue healing sites. The body's natural response to implants is to produce inflammation and ROS. In addition, researchers also prepared pH-sensitive PUR, which can self-assemble to form micelles and is expected to become a multifunctional active intracellular drug transport carrier. In tissue engineering, researchers are developing PEU (Degrapol®) as a scaffold material; in orthopedics, researchers have developed an injectable two-component PUR (PolyNova®) that is used in liquid form under arthroscopy and polymerizes in situ at body temperature to provide appropriate connection and support. The performance it exhibits is equivalent to or better than commonly used bone cement, and it can also promote cell adhesion and proliferation.
Dongguan Fortune Medical Technology Co., Ltd. (Introduction to absorbable polymer materials)
Dongguan Fortune Medical Technology Co., Ltd. is based on medical monomers and medical degradable polymer polyester materials (biomaterials), with interventional non-implantable devices (absorbable devices) as the core, providing customers with integrated, End-to-end high-end consumables R&D and production services continue to lower the R&D threshold for absorbable medical devices and help customers improve R&D efficiency. Bring more breakthrough treatment plans to patients, and the service scope covers the research and development and production of medical monomers, medical degradable polymer polyester materials (biomaterials), medical absorbable monofilaments/multifilaments, and absorbable medical devices.
In terms of polymer degradable materials, our technical core is based on years of accumulation, and we have mature and proven technical reserves. We have become a pioneer and leader in the Chinese absorbable materials market.
-
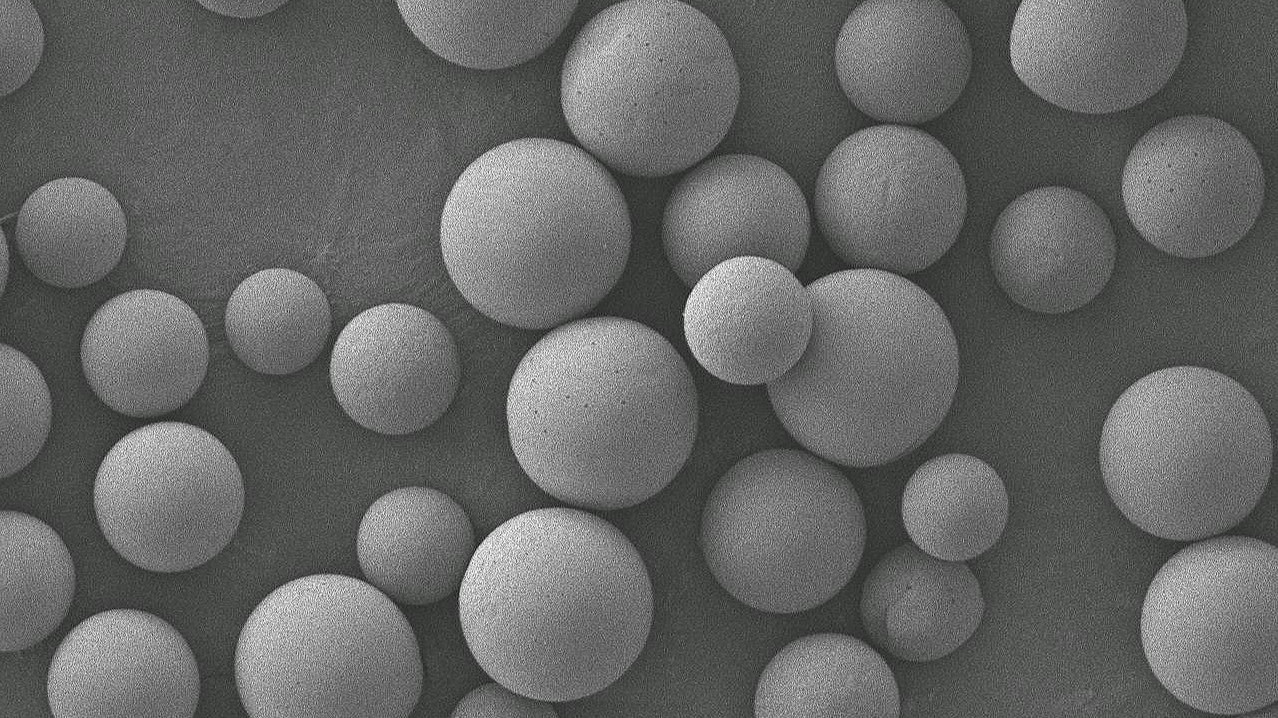
Quantitative Detection Method for Residual Monomer in Polylactic Acid
Polylactic acid has good biocompatibility and degradability, good thermoplasticity and high strength. It can be processed into various medical products. It has been widely used in medical device products such as fracture internal fixation and bone repair, degradable stents, absorbable sutures, tissue engineering stents, 3D printing implants, etc.2025-01-04
-
Application of biodegradable polymer materials in medical devices
The performance and degradation characteristics of several commonly used biodegradable polymer materials are reviewed, including polyglycolide,polylacticacid,(glycolide-lactide) copolymer,polycaprolactone,polydioxanone,polyhydroxyalkanoate, polytrimethylene carbonate, polyurethane and polyether urethane, etc., and their applications in medical devices, including implants, tissue engineering scaffolds, drug controlled release carriers, etc. are reviewed.2025-01-04
-

Method for determination of monomer residues in biodegradable polydioxanone materials
Establish a gas chromatography method for testing the residual monomer dioxanone in polydioxanone raw materials. A DB-624 capillary column (30.0 m ×535 μm ×3.00 μm) was used, and the temperature was programmed (146 ℃ for 5 min, 30 ℃/min to 200 ℃, and maintained for 2 min).2025-01-04






